Editorial by Dr. Seiji Aoyagi
Chief Science Officer, NiHTEK®
- Chief Science Officer at NiHTEK®
- 35+ years in sports & clinical nutrition (Japan)
- Leads NiHPRO®Family (Core, Bev, Puffs) & KARBFiX® science
- Guides APH™ & MPi™ technology programs
- University/hospital collaborations on human studies
- Champion of third-party validation (Informed-Choice®, Informed-Protein®)
- Principal scientific spokesperson for “Protein For EveryBODY™”

In the modern protein industry, purity has become the foundation of scientific credibility in the public health and the wellbeing of those use protein supplements. A landmark New York Times report “Lead Found in Popular Protein Powders and Shakes” (Astor, Oct 14 2025) revealed that more than two-thirds of the most popular protein powders and shakes contained measurable levels of lead (Pb) exceeding the limit that Consumer Reports deems safe for daily use (The New York Times Article →).
Consumer Reports’ corresponding study, “Protein Powders and Shakes Contain High Levels of Lead” (Consumer Reports Article →), showed that several major brands exceeded California’s Proposition 65* threshold of 0.5 µg per day, some by more than 10 times.
These findings really highlight why we need tighter scientific oversight and better standards for sourcing ingredients and purifying protein products.
At NiHTEK®, this isn’t just a box we check—it’s what pushes us forward every day. We’re picky about where our ingredients come from. We use our own hydrolysis tech and run everything through a multi-stage purification process. NiHPRO® doesn’t just meet the standard for purity and safety in protein—it sets a new one, worldwide.
Why does heavy-metal control matter so much?
Heavy metals like lead, cadmium, mercury, and arsenic build up in your body over time.The risks here aren’t just about tech—they hit close to home. We’re talking real stuff: nerve problems, trouble with having kids, messed up metabolism (Jomova et al., 2025). Lead’s the worst of the bunch. It hangs around in your bones, your blood, your tissues—sometimes for years. And honestly, it doesn’t take much to cause harm. That’s why health agencies don’t just set some “okay” limit. They want lead levels as low as humanly possible (that’s the ALARA principle). If there’s lead in your blood, you’ve been exposed. For kids, zero is the goal. There’s no magic number that makes it safe. So really, cut your lead exposure down to nothing if you can, especially for kids and pregnant women.

For athletes and patients consuming high protein quantities daily, even small exposures become significant over time. By achieving ND (not detectable) levels for lead and ultra-low traces for all others, NiHPRO® provides unprecedented assurance for clinicians, nutritionists, and formulators worldwide.
Independent Evidence of Exceptional Purity Across the NiHPRO® Family
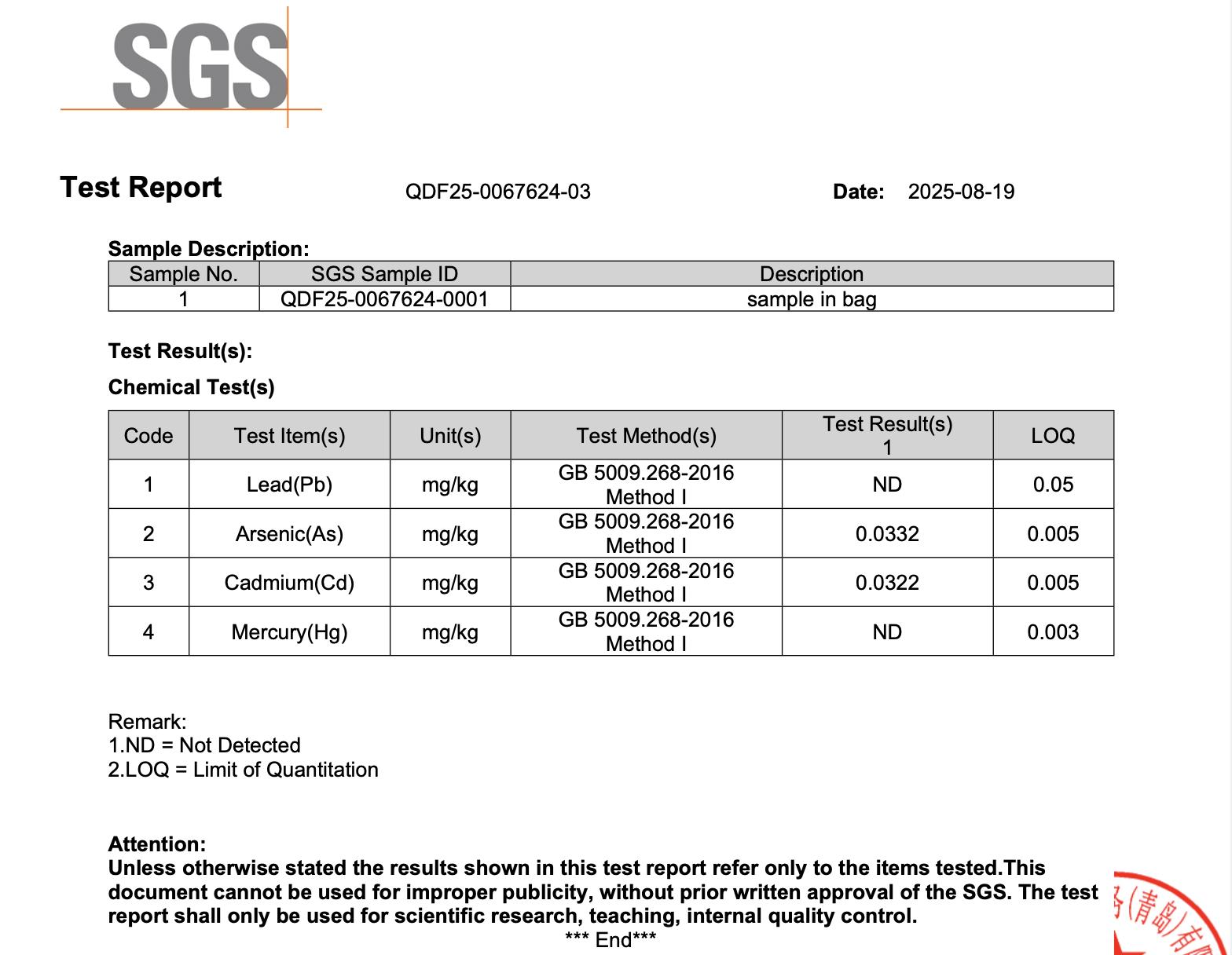
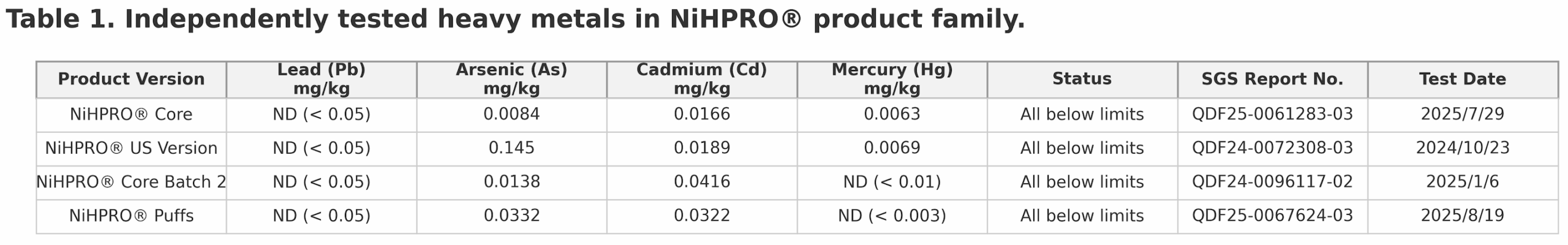
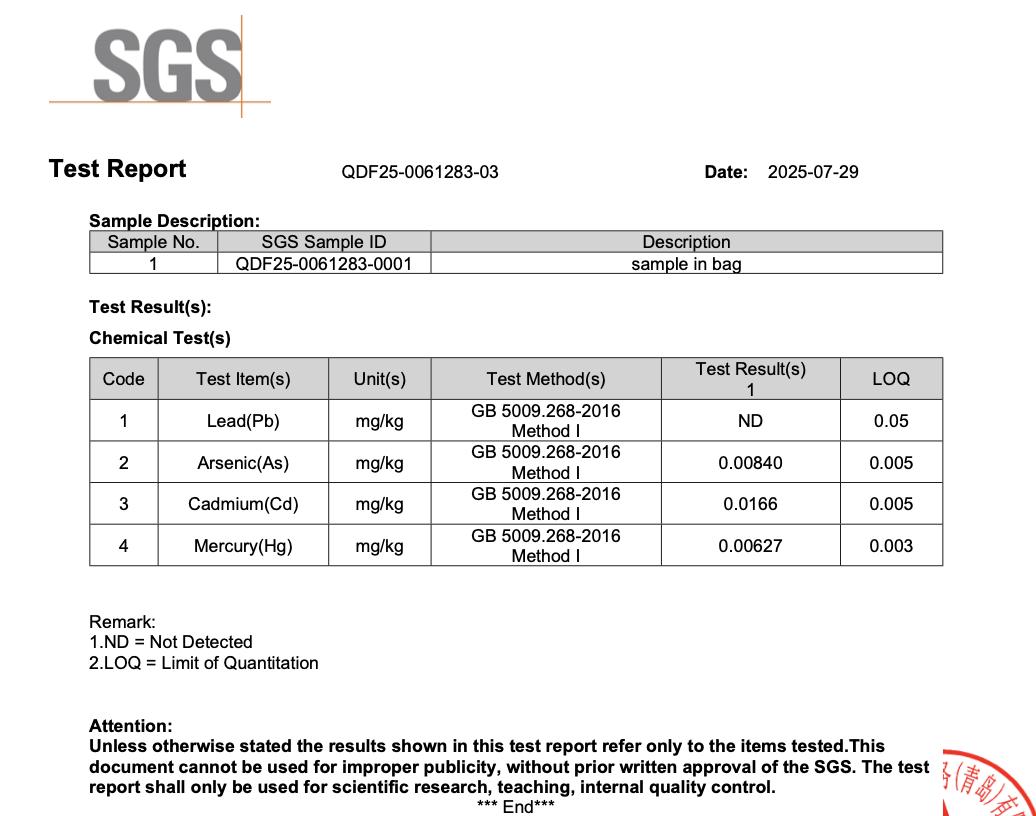
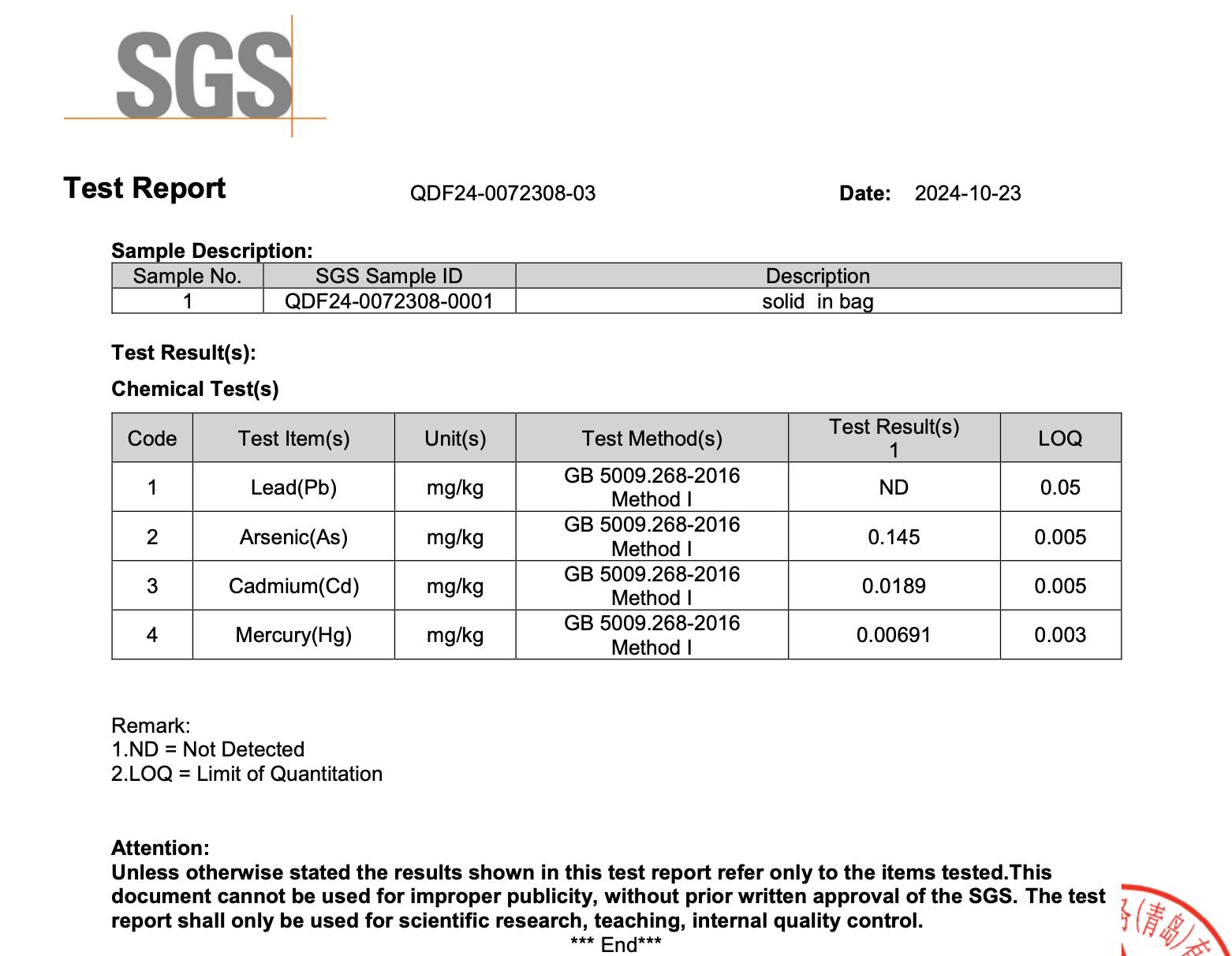
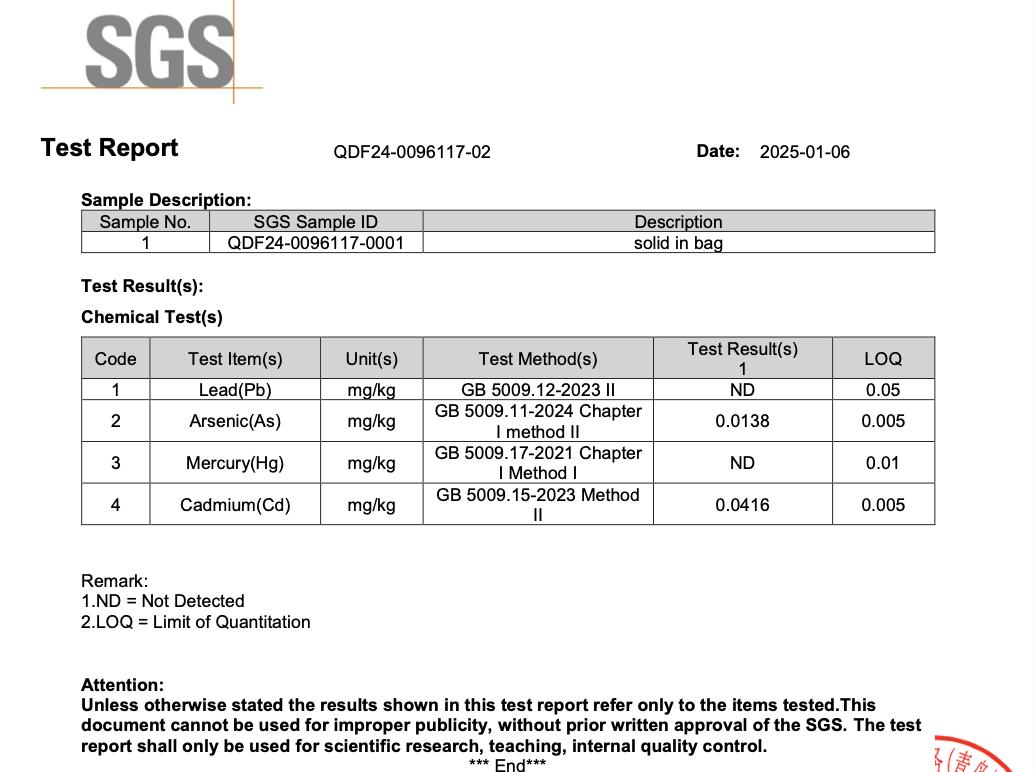
All NiHPRO® batches are tested by SGS, one of the world’s most respected analytical laboratories, following National Food Safety Standard: Determination of Multi-elements in Foods for food-grade heavy-metal detection. Across all four independent SGS tests, lead was not detected in any NiHPRO® sample (Table 1). All other heavy metals remained well below international food safety thresholds, affirming NiHTEK’s commitment to scientific transparency and repeatable excellence.
This means at a 30 g serving size, even the upper detection threshold equates to < 1.5 µg lead per serving, with actual measured value of zero (0). This easily satisfies both California Prop 65 (0.5 µg/day) and U.S. FDA interim guidance (8.8 µg/day for women).
Comparison to Industry Benchmarks
According to the Consumer Reports dataset (as cited in The New York Times), several widely sold “clean” proteins contained detectable levels of lead (Table 2).
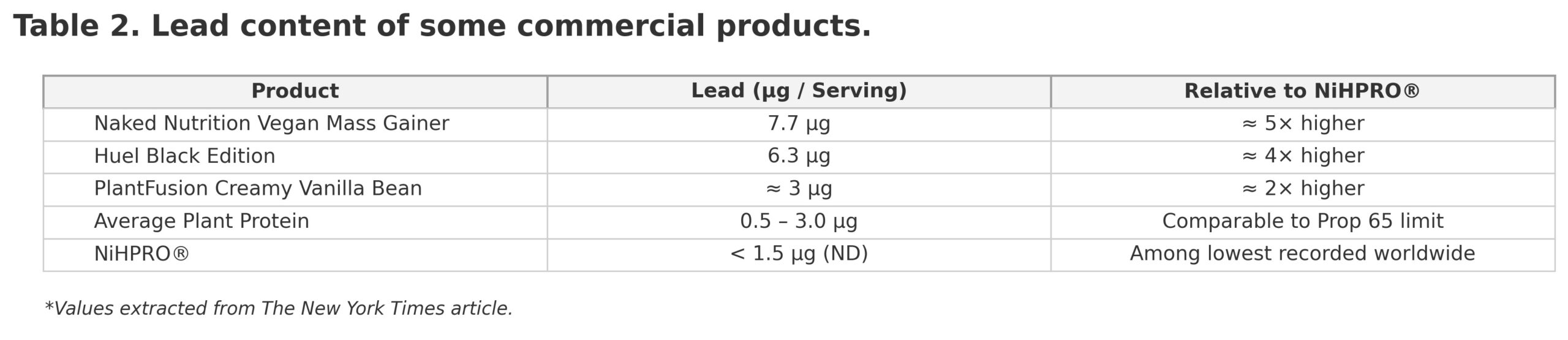
NiHPRO® therefore ranks among the cleanest proteins ever independently tested, significantly purer than both dairy and non-dairy commodity isolates.
The NiHTEK® Sourcing Protocol: Purity Begins at the Source
NiHTEK®’s raw material assurance framework is designed to eliminate contamination risks before processing even begins.
- We get our pea and rice protein isolates straight from farms in Northern China and Australia—places known for clean soil and water, so the risk of contamination stays low.
- Before we accept anything, suppliers have to send us ICP-MS test results that prove heavy metal levels are basically undetectable.
- Every batch is easy to track, right from the field all the way to the final production run.
- We don’t allow any chemical solvents or animal contact—everything stays non-GMO and allergen-free from start to finish.
- All our raw material facilities keep up-to-date ISO 22000 and FSSC 22000 certifications. That means we’re serious about food safety, and we stick to international standards.
This disciplined sourcing ensures that clean inputs enter an equally clean, sealed processing system.
Patent-Pending Purification and Hydrolysis Technologies
Advanced Precision Hydrolysis™ (APH™)
This is our proprietary enzymatic platform. It pinpoints specific peptide bonds, so you get better digestibility and higher bioavailability. NiHTEK® keeps everything under tight computer control — pH, temperature, all of it — to steer clear of any unwanted by-products. We never use chemical solvents, and the reactors stay fully sealed. That way, nothing leaks out, nothing pollutes.
Molecular Protein Infusion™ (MPi™)
Here’s our multi-stage purification system. First, it runs the material through activated carbon and ultrafiltration to clear out color, odor, and any leftover residue. Then, inline ion-exchange strips out trace metals so thoroughly, you can’t even measure what’s left. Finally, vacuum-dry crystallization stabilizes the protein and locks in its purity.
Together with NiHTEK®’s new patent-pending Purification Enhancement Sequence, these systems produce a pharma-grade protein matrix with consistent “Not Detected” lead results across all batches.
NiHPRO® sets a new global benchmark for safety and purity in functional proteins. Its multi-batch SGS verification, patent-pending technologies, and clean sourcing framework prove that advanced science can achieve both performance and purity.
“Our commitment at NiHTEK® is not just to meet standards, it is to create them. Through disciplined sourcing, patent-pending purification, and hydrolysis precision, NiHPRO® represents the future of safe nutrition.”
— Dr. Seiji Aoyagi, Ph.D.
Chief Science Officer, NiHTEK®

References
- Astor, M. (2025, Oct 14). Lead Found in Popular Protein Powders and Shakes, Report Says.The New York Times.https://www.nytimes.com/2025/10/14/well/lead-protein-powder.html
- Consumer Reports (2025). Protein Powders and Shakes Contain High Levels of Lead.https://www.consumerreports.org/lead/protein-powders-and-shakes-contain-high-levels-of-lead-a4206364640/
- California OEHHA Proposition 65 Lead Exposure Limits (2024).
- Jomova, K., Alomar, S.Y., Nepovimova, E. et al.Heavy metals: toxicity and human health effects. Arch Toxicol 99, 153–209 (2025).
- S. FDA Interim Reference Levels for Lead (2022).
- NiHTEK® Internal Purification and Hydrolysis Protocols (2025, Patent Pending).
- Appendix 1: SGS Test Report QDF25-0061283-03 (2025-07-29)
- Appendix 2: SGS Test Report QDF24-0072308-03 (2024-10-23)
- Appendix 3: SGS Test Report QDF24-0096117-02 (2025-01-06)
- Appendix 4: SGS Test Report QDF25-0067624-03 (2025-08-19)
Appendix 1: SGS Test Report QDF25-0061283-03 (2025-07-29)
Appendix 2: SGS Test Report QDF24-0072308-03 (2024-10-23)
Appendix 3: SGS Test Report QDF24-0096117-02 (2025-01-06)
Appendix 4: SGS Test Report QDF25-0067624-03 (2025-08-19)