The global nutrition industry is entering a new phase one defined not by commodity ingredients, but by technology-driven, science-validated branded systems. At the center of this shift is NiHTEK®, an Innovative Health Technology Company™, and its flagship protein platform:
NiHPRO®Family alongside the rapid global expansion of KARBFiX®, NiHTEK®’s advanced carbohydrate technology.
In 2025, NiHPRO® is no longer an emerging concept. It is being launched across major global markets and adopted by established brands that demand efficacy, sensory performance, scientific credibility, and supply reliability.
This is not incremental growth.
It is a category-level takeover.
NiHPRO® Is Now Live in Major Global Markets

NiHPRO® powered products are already launching with respected regional and international brands across Asia-Pacific, Europe, Africa, and the UK.
Current Brand Launches
- International Protein – Australia
- Day1 Performance – Australia
- Nutritech Nutrition – Belgium (Europe)
- HARD BODY – Europe
- BIOGENA – Austria
- It’s All Good – South Africa
- NUTRITECH – South Africa
- MyWellness – South Africa
- NATURIXR – Algeria
- StromSports – United Kingdom
- CleverPRO – Japan
Each of these brands has independently chosen NiHPRO®Family as the core protein technology behind their next-generation formulations.
Why Global Adoption Is Accelerating So Fast
NiHPRO® is not winning on hype. It is being adopted because it solves real, long-standing industry problems.
Branded Ingredient Technology Not Commodity Protein

Unlike raw commodity proteins, NiHPRO®Family is engineered as a Functional Branded Ingredient meaning it is built as a scalable technology platform, not simply a raw material.
NiHTEK® integrates:
- Advanced Precision Hydrolysis™ (APH™)
- Molecular Protein Infusion™ (MPi™)
- Precision amino acid balancing
- Application-specific system design
This approach aligns with modern protein science: digestibility, amino acid availability, and formulation performance matter as much as protein grams alone.
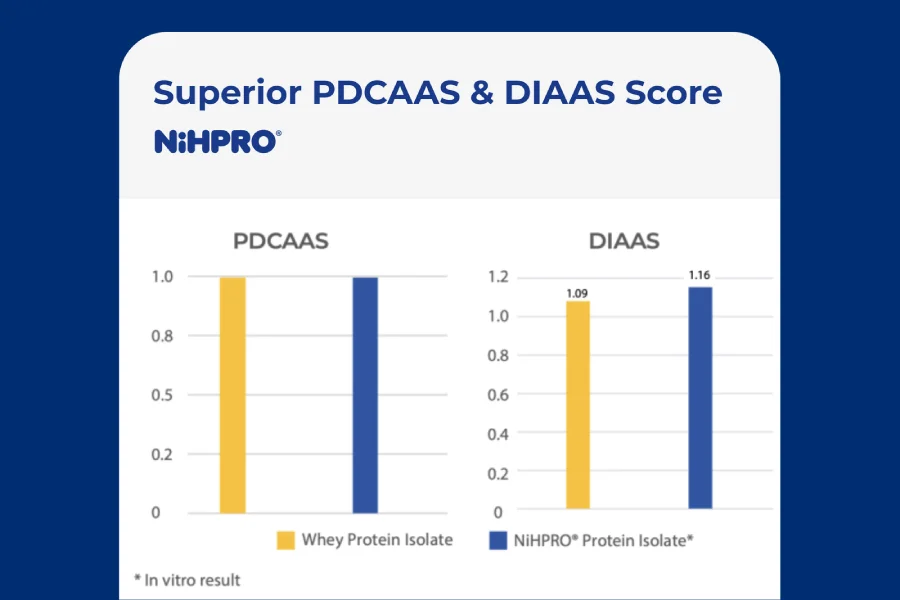
Science-Based Efficacy, Not Marketing Claims
Protein quality is increasingly evaluated using recognized scientific frameworks such as:
- PDCAAS (Protein Digestibility-Corrected Amino Acid Score)
- DIAAS (Digestible Indispensable Amino Acid Score)
The FAO has recommended DIAAS as a more advanced method for evaluating protein quality because it measures amino-acid-specific digestibility.
NiHPRO®Family is positioned to align with these higher standards offering measurable protein quality without relying on vague marketing language.
Verified Quality Through Third-Party Testing
Global brands require proof not promises.
NiHPRO® ingredients are supported by:
- Independent third-party laboratory testing
- Verified amino acid profiles
- Digestibility and purity assessments
- Ongoing quality assurance across production lots
This level of validation is becoming essential as regulators and consumers scrutinize protein quality, contamination, and labeling accuracy.
Global PhD-Led R&D and Application Science
NiHTEK® operates with a global scientific network spanning:
- Protein chemistry
- Digestion physiology
- Food engineering
- Sensory science
- Clinical and functional nutrition
This allows the NiHPRO®Family to scale across multiple formats:
- Powders
- Functional and medical beverages
- Bars, snacks, bites, and cereal inclusions
Few ingredient companies combine deep science with real-world application performance at this scale.
A Truly New Category Nothing Like It Exists

NiHPRO® does not compete head-to-head with legacy protein categories.
It creates a new one.
Not dairy.
Not commodity plant protein.
Not a flavour-masked isolate.
Not a regional novelty.
NiHPRO®Family is a globally scalable protein technology platform built for the next decade of nutrition innovation.
KARBFiX®: Expanding in Parallel Across Global Markets
Alongside NiHPRO®, KARBFiX® is launching with brands across many of the same regions — and more.
KARBFiX® is NiHTEK®’s advanced carbohydrate solution designed for:
- Clean energy delivery
- Digestive comfort
- High solubility and clarity
- Allergen-free, non-GMO formulations
Together, NiHPRO®Family + KARBFiX® give brands the ability to build complete, performance-driven nutrition systems protein and carbohydrate technologies designed to work in parallel.
What’s Launching Next: The Next 3-4 Months

NiHTEK® confirms that major globally recognized brands are preparing launches powered by:
- NiHPRO®Core
- NiHPRO®Bev
- NiHPRO®Puffs
- KARBFiX®
Confirmed Expansion Regions
- United States
- Germany
- India
- Japan
- Australia
- New Zealand
- South Africa
- United Kingdom (England & Ireland)
- Israel
- Dubai (UAE)
These launches will span powders, beverages, snacks, bars, and cereal formats marking one of the fastest global rollouts of a branded protein ingredient family in industry history.
Why Brands Are Switching to NiHTEK®

In simple terms, brands are choosing NiHTEK® because it offers:
- Cutting-edge food and nutrition technology
- Science-based efficacy aligned with global standards
- Third-party verified quality and safety
- Application-ready performance
- Global scalability
- Stable and competitive pricing
- A category-creating story not a commodity race
FAQ
Is NiHPRO® a protein powder?
No. NiHPRO®Family is a branded protein ingredient platform used inside finished products.
Why are so many regions launching at once?
Because NiHPRO® solves universal formulation challenges taste, digestion, and performance while remaining globally scalable.
Can NiHPRO® be used in snacks and beverages?
Yes. NiHPRO®Core, NiHPRO®Bev, and NiHPRO®Puffs are designed for different application formats across food and beverage systems.
References
- FAO. Dietary Protein Quality Evaluation in Human Nutrition (2013).
- U.S. FDA. Food Allergen Labeling and Consumer Protection Act (FALCPA) overview.
- Loveday SM. Protein digestion and absorption: influence of food processing. Nutrition Research Reviews.
Disclaimer
This article is for general informational purposes only and does not constitute medical, regulatory, or legal advice. Product claims, labeling, and compliance must be validated for each finished product and market.





